- Visibility 194 Views
- Downloads 32 Downloads
- Permissions
- DOI 10.18231/j.jpmhh.2021.022
-
CrossMark
- Citation
Physico-chemical study of Panchvalkal mentioned in Himvan Agada: An anti — Poisonous formulation in Ayurveda
- Author Details:
-
Parvesh Kumar *
-
Rudramani Deepak
-
Dharni Dhar Singh
-
Rajeev Ranjan
Abstract
Agadatantra is one among the Ashtangas of Ayurveda, which deals with all cases of poisoning.
In all these situations, Sarpvisha is of paramount importance because it is a condition that requires emergency management. Himavanagada is one among them. Ayurveda has grouped the stem bark of five plants viz. Nyagrodha (Ficus bengalensis Linn.), Shirish (Albizzia lebbeck Benth.), Ashwatha (Ficus religiosa Linn) Vetasa (Salix caprea Linn.) and Plaksha (Ficus infectoria Roxb.) as ingredients in Himavan agada (the stem bark of five trees). Itcontains 14 ingredients and indicated for mandalivisha, visarpa, shwayathu, visphota, jwara and daha.
Objectives: To analyze the Physico-chemical, phyto- chemical properties.
Materials and Methods: Procurement of Raw drugs was later subjected to Physico-chemical analysis and preliminary phytochemical analysis.
Conclusion: All the parameters, assays are done, upon which the standards of Pharmacopoeia depend.
Introduction
Ayurveda has grouped the stem bark of five plants viz. Nyagrodha (Ficus bengalensis Linn.), Shirish (Albizzia lebbeck Benth.), Ashwatha (Ficus religiosa Linn) Vetasa (Salix caprea Linn.) and Plaksha (Ficus infectoria Roxb.) as ingredients in Himavan agada (the stem bark of five trees).[1]
The Himavan agada are indicated as Varnya (improve complexion), Stanyashodhana (purifies breast milk), Vranaghna (wound healing), Visarpa (for treating Herpes zooster) and Shophaghna (anti-inflammatory) identified by Arundatta the commentator of Ashtanga Hrudaya. [2] Also Himavan agada (anti-poisonous formulation) used to treat the Mandali Sarpa Visha.
Therefore the present study has been planned to explore and analyze the poly herbal combination were subjected to Physico-Phytochemical analysis of Panchvalkal mentioned in Himvan agada.
Aims and Objective
To analyze the Physico-chemical, phyto- chemical properties of Himvan Agada by using HPTLC fingerprinting.
Materials and Methods
1. Preparation of Himavan agada
Raw drugs required for preparation were procured form authenticated market dealer. Procured drugs were authenticated in Department of Dravyaguna, Gaur Brahman Ayurvedic College, Rohtak. Drugs were pounded individualy in Khalwa Yantra to get Yawakuta form. After pounding drugs are mixed (I part each) to prepare Himavan agada. Ingredient of Himavan agada is mentioned in table.
|
Sl no |
Name of Ingredients |
Quantity |
|
1. |
Nyagrodha (Ficus bengalensis Linn.) |
1 part |
|
2. |
Shirish (Albizzia lebbeck Benth.) |
1 part |
|
3. |
Asvattha (Ficus religiosa Linn.) |
1 part |
|
4. |
Vetasa (Salix caprea Linn.) |
1 part |
|
5. |
Plaksha (Ficus infectoria) |
1 part |
Determination of ash value/total ash [3]
Description
The residue remaining after incineration is the ash content of the drug, which represents inorganic salts. Ash value is a criterion to judge the identity or purity of crude drug. This test is applicable for Solid, Semi-solid dosage form.
Equipment and glassware used
Muffle-furnace, Silica Crucible, Desiccator, Ash less filter paper, Beaker.
Reagent/ chemicals used
NA
Procedure
Incinerate about 2 to 3 gm of drug (W1) accurately weighed and ground in a tarred silica crucible (W2). Keeps the crucible in a muffle-furnace at a temperature not exceeding 450˚C- 600˚C (According to the physical property of product) for 4 hrs. Cool in desicator and weigh (W3). If a carbon free ash cannot be obtained in this way. After cooling reignite the crucible at same temp. for 01 hrs. and calculated the weight difference. Collect the residue on an ash less filter paper. Incinerate the residue and filter paper. Add the filtrate, evaporate to dryness. Ignite at a temperature not exceeding 450˚C - 600˚C. Calculate the percentage of ash with reference to the air-dried drug.
Calculation
Percentage of Ash:=W3-W2 X 100 W1Where, W3= Weight of crucible with ash, W2= Weight of crucible and W1= Weight of drug taken.
Determination of acid insoluble ash [4]
Description
The residue remaining after incineration is the ash content of the drug, which represents inorganic salts. Ash value is a criterion to judge the identity or purity of crude drug. This test is applicable for Solid, Semi-solid dosage form.
Equipment and glassware used
Muffle-furnace, Silica Crucible, Desiccator, Ash less filter paper, Beaker.
Reagent/ chemicals used
Dil. hydrochloric acid
Procedure
Prepare ash. Transfer the ash in a 250 ml beaker without loss of ash and add 100 ml of dil. hydrochloric acid. Wash the crucible with 10 ml of acid and transfer the washings to the beaker. Heat the beaker till the liquid boils. Filter the solution and collect the insoluble matter on ash less filter paper (Whatman). Wash with hot water until the filtrate is neutral. Transfer the filter paper containing the insoluble matter to the original crucible. Dry on a hot plate and ignite at 600˚C in a muffle furnace (until become white ash). Allow the residue to cool in suitable desiccators for 30 minutes and weigh without delay. Repeat the process until constant weight (W3) is obtained. Calculate the acid insoluble ash with reference to the air dried drug.
Calculation
Percentage of Acid insoluble Ash:W3-W2 X 100W1Where, W3= Weight of crucible with ash, W2= Weight of crucible, W1= Weight of drug taken.
Determination of water soluble ash [5]
Description
The residue remaining after incineration is the ash content of the drug, which represents inorganic salts. Ash value is a criterion to judge the identity or purity of crude drug. This test is applicable for Solid, Semi-solid dosage form.
Equipment and glassware used
Muffle-furnace, Silica dish, Desiccators, Ash less filter paper, Beaker.
Reagent/ chemicals used
Purified Water
Procedure
Prepare ash. Boil the total ash for 5 minutes with 25 ml of water. Collect insoluble matter in an ash less filter paper. Wash with hot water and ignite for 15 minutes at a temperature not exceeding 450°-600˚C. Subtract the weight (W3) of the insoluble matter from the weight of the ash; the difference in weight represents the water-soluble ash. Calculate the percentage of water- soluble ash with reference to the air-dried drug.
Calculation
Percentage of Water insoluble Ash:(W3-W2) X 100W1Where, W1= Weight of drug taken, W2= Weight of empty crucible and W3= Weight of crucible+ water insoluble ash.
Water soluble ash = Total ash — Water Insoluble Ash
Determination of alcohol soluble extractive[6]
Description
The extracts obtained by exhausting crude drugs are indicative of approximate measures of their chemical constituents. Taking into consideration the diversity in chemical nature and properties of contents of drugs, various solvents are used for determination of extractives. The solvent used for extraction is in a position to dissolve appreciable quantities of substances desired.
Equipment and glassware used
Hot air oven, Flask, Flat bottom shallow dish
Reagent/ chemicals used
Alcohol
Procedure
Macerate 5 gm (W1) of the coarsely powdered drug with 100 ml of Alcohol of specified strength in a closed flask for 24 hours. Shake frequently during six hours. Allow to stand for eighteen hours. Filter rapidly taking precautions against loss of solvent. Evaporate 25 ml of the filtrate to dryness in a tarred flat bottom shallow dish (W2). Dry at 1050C to constant weight and weigh (W3). Calculate the percentage of Alcohol– soluble extractive with reference to the air-dried drug.
Calculation
Percentage of Alcohol soluble extractive:(W3-W2) X100X100W1 X 25Where, W1= Weight of drug taken, W2= Weight of empty dish and W3= Weight of dish+extractive residue.
Determination of water soluble extractive[7]
Description
The extracts obtained by exhausting crude drugs are indicative of approximate measures of their chemical constituents. Taking into consideration the diversity in chemical nature and properties of contents of drugs, various solvents are used for determination of extractives. The solvent used for extraction is in a position to dissolve appreciable quantities of substances desired.
Equipment and glassware used
Hot air oven, Flask, Flat bottom shallow dish
Reagent/ chemicals used
DM. Water
Procedure
Macerate 5 gm (W1) of the coarsely powdered drug with 100 ml of distilled water in a closed flask for 24 hours. Shake frequently during six hours. Allow to stand for eighteen hours. Filter rapidly taking precautions against loss of water. Evaporate 25 ml of the filtrate to dryness in a tarred flat bottom shallow dish (W2). Dry at 1050C to constant weight and weigh (W3). Calculate the percentage of water – soluble extractive with reference to the air-dried drug.
Calculation
Percentage of Water soluble extractive:W3-W2X100X 100W1 X 25Where, W1= Weight of drug taken, W2= W2ght of empty dish and W3= Weight of dish+extractive residue.
Loss on drying (Oven Method)[8]
Description
Moisture content is the amount of moisture present in a crude drug sample. It should be minimized in order to prevent decomposition of crude drugs either due to chemical change or microbial contamination.
Equipment and glassware used
Hot air oven, LOD Bottle, Petri Dish
Reagent/ chemicals used
NA
Procedure
(Oven Method)
Sample is prepared by cutting shredding so that the parts are about 3 mm in thickness. Place about 10 gm (W1) accurately weighed up to third decimal place of drug (Without preliminary drying) in a tarred evaporating dish + drug weight (W2). Dry at 105˚C for 5 hours and weigh (W3). Continue the drying and weighing at one hour interval until difference between two successive weighing corresponds to not more than 0.25 percent. (Constant weight is reached when two consecutive weighing after drying for 30 minutes). Cool for 30 minutes in desiccator, show not more than 0.001 g difference.
|
Sl. no |
Parameter |
Reagent preparation |
Observation |
|
1. |
Tests for Alkaloids |
||
|
A. |
Dragendroff’s test |
Bismuth sub nitrate- 1.7 gm Glacial Acetic acid- 20 ml Pott. Iodide (50%in water)- 100 ml Water- 80 ml |
Alcoholic extract acidified with 2 M HCl + Reagent= Reddish brown precipitate |
|
B. |
Mayer’s test |
Mercuric Chloride- 1.36 gm Pott. Iodide- 5 gm Water- 100 ml |
Alcoholic extract+Reagent= cream precipitate |
|
C. |
Wagner’s test |
Iodine- 2 gm Pott. iodide- 6 gm Water- 100 ml |
Alcoholic extract acidified with 1.5% HCl + Reagent = Yellow or brown precipitate |
|
D. |
Hager’s test |
Picric Acid- 1.0 gm Water- 100 ml |
Alcoholic extract + Reagent = yellow precipitate |
|
2. |
Tests for Glycosides |
||
|
A. |
Keller-killiani Test (for deoxy sugar): |
1 ml of glacial acetic acid containing traces of FeCl3 and 1 ml of conc. H2SO4 were added. |
Aqueous extract + Reagent=A reddish-brown colour formed at the junction of two layers and the upper layer turned bluish green. |
|
B. |
Legal test (for cardinolides) |
Conc. Ethanolic extract was made alkaline with few drops of 10% NaOH and then add freshly prepared Sod. Nitroprusside solution. |
Conc ethanolic extract + Reagent= Blue coloration observed. |
|
C. |
Baljet test |
Picric acid is added to the extract and made alkaline. |
Aqueous extract + Reagent= Stable orange color. |
|
D. |
Borntrager’s test |
Dil. HCl is added to powdered drug and heated for 5 mins then filtered and add equal volume of CHCl3, shake well and collect the lower org. layer of CHCl3, add NH3 half of its volume, shake well. |
Aqueous extract or powder drug + Reagent= Lower Ammonical layer turned rose pink colour. |
|
3. |
Tests for Saponins |
||
|
A. |
Foam Test: |
Powdered drug residue was taken in a test tube and shaken vigorously with a small amount of NaHCO3 and water. |
Drug + Reagent= Characteristic honeycomb like froth obtained. |
|
4. |
Tests for Steroids |
||
|
A. |
Salkowaski reaction |
Residue of extract taken in 2 ml of CHCl3 and 2 ml of conc. H2SO4 added from the side of the test-tube and then shaken for few minutes. |
Alcoholic extract + Reagent= Red colour developed in the CHCl3 layer. |
|
B. |
Libberman Burchard’s Test |
Acetic Anhydride Sulphuric acid |
2mg of extract was dissolved in acetic anhydride, boil and cool then add 1 m conc H2SO4. Formation of pink colour. |
|
5. |
Tests for Tannins and Phenolic Compounds |
||
|
A. |
Ferric chloride reagent |
5% FeCl3 sol. added to test sol. |
Aq. or alcoholic ext + Reagent= Dark green or deep blue colour is obtained. |
|
B. |
Lead acetate test |
A 10% w/v solution of basic lead acetate in distilled water was added to test sol. |
Aq. extract +Reagent= Precipitation occurred. |
|
C. |
Gelatin solution tes. |
1% w/v solution of gelatin in water, with 10% sodium chloride and then added to test sol. |
Aq. extract + Reagent= White precipitate is obtained |
|
6. |
Tests for Flavonoids |
||
|
A. |
Shinoda Test: |
Test residue dissolved in 5 ml ethanol (95% v/v) and reacted with few drops of conc. HCl and 0.5 g of Mg metal. |
Hydro-alcoholic or alcoholic extract + Reagent= The pink, crimson or magenta colour is developed. |
|
B. |
Ammonia Test: |
Filter paper strips were dipped in the alcoholic extract and ammoniated. |
Alcoholic extract + Reagent= Strips turned yellow |
Calculation
Percentage of loss on drying: W2-W3 X 100W1Where, W1= Weight of drug taken, W2= Weight of evaporating dish + drug before drying and W3= Weight of evaporating dish + drug after drying.
Thin layer chromatography (TLC) test[10]
Description
TLC is the technique in which a solute undergoes distribution between two phases, stationary phase and a mobile phase. The stationary phase acts as an adsorbent in a relatively thin uniform layer of a dry finely powdered material applied to a glass, plastic or metal sheet.
Separation may be achieved on the basis of partition a or a combination of partition and adsorption, depending on the particular type of stationary phase, its preparation and uses of different solvents.
Equipment and glassware used
Oven, TLC developing chamber, Visualizing chamber, Pre-coated aluminium plates with silica gel 60 F254, Beaker, measuring cylinder with stoppered and glass pipettes.
Reagent/ chemicals used
Different solvents and post-derivatise reagents.
Conditions
Stationary phase: Pre-coated silica gel 60 F254 aluminium plates
Mobile phase: Toluene: Ethyl acetate: Formic acid (8:2:0.1).
Chamber Saturation Time: 20 mins.
Test Solution: Dissolve 500 mg of sample in 10 ml of methanol.
Derivatising Agent- 5% Sulphuric Acid in Methanol
HIGH performace thin layer chromatography (TLC) test[11]
HPTLC is the technique in which a solute undergoes distribution between two phases, stationary phase and a mobile phase. The stationary phase acts as an adsorbent in a relatively thin uniform layer of a dry finely powdered material applied to a glass, plastic or metal sheet.
Separation may be achieved on the basis of partition a or a combination of partition and adsorption, depending on the particular type of stationary phase, its preparation and uses of different solvents.
Instrument contains different parts to applicate, scan and visualize the elution of different spots after development of TLC plate. So, we can easily develop a fingerprint profile for the same.
Equipment and glassware used
Linomats TLC applicator, Oven, TLC developing chamber, Visualizing chamber, Pre-coated aluminium plates with silica gel 60 F254, Beaker, measuring cylinder with stoppered and glass pipettes, Camag TLC scanner and photographic chamber.
Reagent/ chemicals used
Different solvents and post-derivatise reagents.
Observation and Results
|
Sl.No. |
Parameters |
Sample |
|
1. |
Alcohol Soluble Extractive |
7.62% |
|
2. |
Water Soluble Extractive |
15.26% |
|
3. |
LOD |
10.29% |
|
4. |
Total Ash |
8.16% |
|
5. |
Water Soluble Ash |
2.55% |
|
6. |
Acid Insoluble Ash |
0.91% |
Organoleptic character
|
Sr. No. |
Parameters |
Panchavalka |
|
1 |
Appearance |
Crude raw drug |
|
2 |
Odour |
Characteristic |
|
3 |
Taste |
- |
|
4 |
Colour |
Brownish |
Qualitative parameters
|
Sl. no |
Qualitative chemical Test |
Results |
Method |
|
1. |
Alkeloids |
Negative |
API 2008 |
|
2. |
Glycosides |
Positive |
|
|
3. |
Tannins |
Positive |
|
|
4. |
Flavonides |
Positive |
|
|
5. |
Saponins |
Negative |
|
|
6. |
Steroids |
Negative |
|
Test Parameter |
Limits |
Result |
Test Method |
|
TLC Profile Sample |
254nm |
0.68 |
API 2008 |
|
366nm |
0.93,0.82,0.62,0.4,0.2 |
||
|
Derivatized |
0.93, 0.82, 0.75, 0.70, 0.5, 0.31 |
|
|
Test Parameter |
Limits |
Result |
|
HPTLC Fingerprinting Profile sample B |
254 nm |
0.18, 0.29, 0.54, 0.57, 0.64 |
|
310 nm |
0.18, 0.54, 0.58, 0.66, 0.68 |
|
|
|
366 nm |
0.18, 0.34, 0.54, 0.58, 0.65, 0.66, 0.7, 0.72, 0.79 |
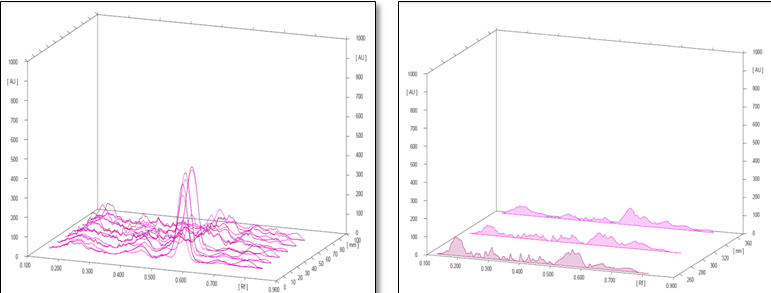
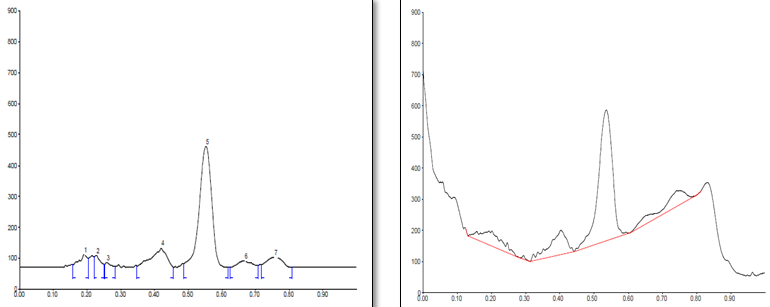
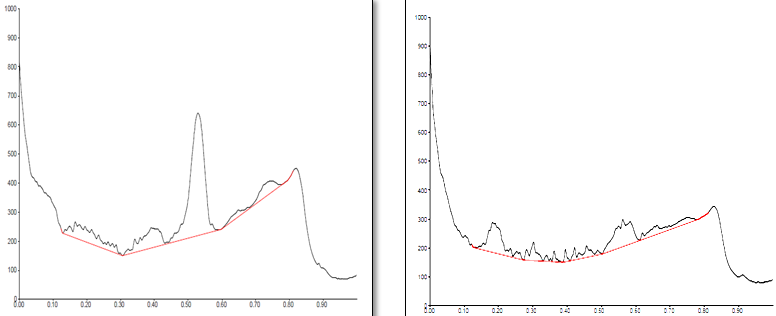
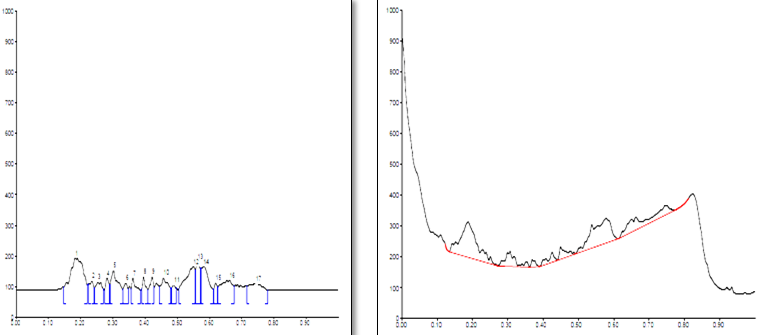
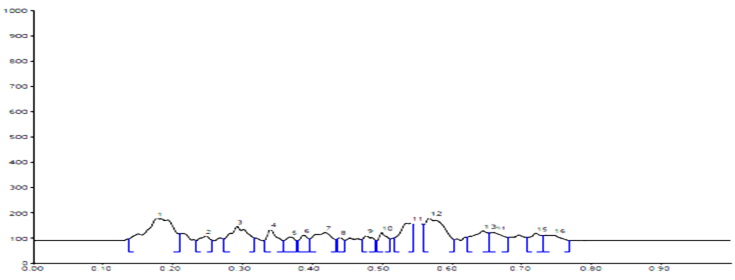
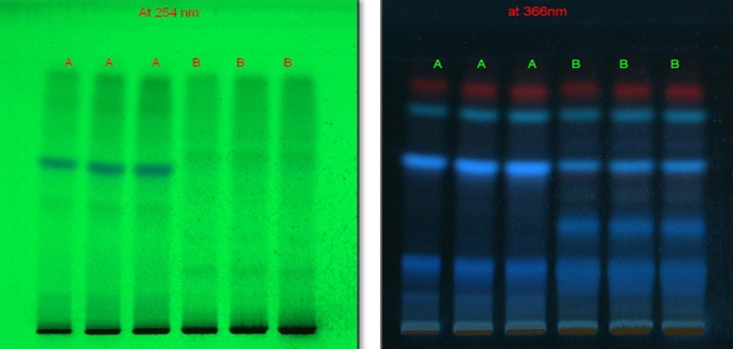
Conditions
Stationary phase: Pre-coated silica gel 60 F254 aluminium plates
Mobile phase: Toluene: Ethyl acetate: Formic acid (5:4:1).
Chamber Saturation Time: 20 mins.
Test Solution: Dissolve 100 mg of sample in 10 ml of methanol.
Derivatising Agent- Anisaldehyde Sulphuric Acid
Discussion & conclusion
HPTLC fingerprinting profile
The different concentration of Methanolic extract of samples was applied on HPTLC plates in 6, 8 and 10 µl. Plate was developed and elution of different spots visualized under 254, 366nm and white light after derivatization with Anisaldehyde sulphuric acid reagent.
Physico-phytochemical analysis
The quantitative tests e.g. total ash, acid-insoluble ash, water-soluble ash, alcohol-soluble extractive, water- soluble extractive, ether-soluble extractive, moisture content, volatile oil content and assays are the methods upon which the standards of Pharmacopoeia depend. So it is necessary to analyze Ayurvedic formulation on these parameters.
Source of Funding
None.
Conflict of Interest
None.
References
- TH. Madanapala Nighantu. . 1998. [Google Scholar]
- TY. Agnivesha, Charaka samhita. . 2009;1:23-23. [Google Scholar]
- . Ayurvedic pharmacopoeia of India-2001, part1, vol-1st, appendices 2.2.3 Pg.143.. . . [Google Scholar]
- . Ayurvedic pharmacopoeia of India, 2001, part1, vol-I, appendices 2.2.4, Pg.143.. . . [Google Scholar]
- . The Ayurvedic pharmacopoeia of India, 2001, part 1, Vol-I, appendix 2.2.5, pg.143.. . . [Google Scholar]
- . The Ayurvedic pharmacopoeia of India, 2001, part 1, Vol-I, appendix 2.2.5, pg.143.. . . [Google Scholar]
- . The Ayurvedic pharmacopoeia of India, 2001, part1, Vol-I, appendix 2.2.7, pg. 143.. . . [Google Scholar]
- . Ayurvedic pharmacopoeia of India, 2001, Part1, vol-I, appendix 2.2.9, pg.143.. . . [Google Scholar]
- . Ayurvedic pharmacopoeia of India, 2011, Part1, vol-VIII, Pg no-220, appendices 3.5, Govt. of India, New Delhi. . . [Google Scholar]
- Farooqui NA, Dey A, Singh GN, Easwari TS, Pandey MK. Analytical techniques in quality evaluation of herbal drugs.. Asian J Pharm Res. 2011;4(3):112-7. [Google Scholar]
- . Khandelwal KR.Practical pharmacognosy.19th ed. Pune. Nirali prakashan;2009.pg.149-56. . . [Google Scholar]
- Abstract
- Introduction
- Aims and Objective
- Materials and Methods
- Determination of ash value/total ash [3]
- Equipment and glassware used
- Reagent/ chemicals used
- Determination of acid insoluble ash [4]
- Equipment and glassware used
- Reagent/ chemicals used
- Determination of water soluble ash [5]
- Equipment and glassware used
- Reagent/ chemicals used
- Determination of alcohol soluble extractive[6]
- Equipment and glassware used
- Reagent/ chemicals used
- Determination of water soluble extractive[7]
- Equipment and glassware used
- Reagent/ chemicals used
- Loss on drying (Oven Method)[8]
- Equipment and glassware used
- Reagent/ chemicals used
- Thin layer chromatography (TLC) test[10]
- Equipment and glassware used
- Reagent/ chemicals used
- HIGH performace thin layer chromatography (TLC) test[11]
- Equipment and glassware used
- Reagent/ chemicals used
- Observation and Results
- Discussion & conclusion
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Kumar P, Deepak R, Singh DD, Ranjan R. Physico-chemical study of Panchvalkal mentioned in Himvan Agada: An anti — Poisonous formulation in Ayurveda [Internet]. J Prev Med Holist Health. 2021 [cited 2025 Sep 29];7(2):114-120. Available from: https://doi.org/10.18231/j.jpmhh.2021.022
APA
Kumar, P., Deepak, R., Singh, D. D., Ranjan, R. (2021). Physico-chemical study of Panchvalkal mentioned in Himvan Agada: An anti — Poisonous formulation in Ayurveda. J Prev Med Holist Health, 7(2), 114-120. https://doi.org/10.18231/j.jpmhh.2021.022
MLA
Kumar, Parvesh, Deepak, Rudramani, Singh, Dharni Dhar, Ranjan, Rajeev. "Physico-chemical study of Panchvalkal mentioned in Himvan Agada: An anti — Poisonous formulation in Ayurveda." J Prev Med Holist Health, vol. 7, no. 2, 2021, pp. 114-120. https://doi.org/10.18231/j.jpmhh.2021.022
Chicago
Kumar, P., Deepak, R., Singh, D. D., Ranjan, R.. "Physico-chemical study of Panchvalkal mentioned in Himvan Agada: An anti — Poisonous formulation in Ayurveda." J Prev Med Holist Health 7, no. 2 (2021): 114-120. https://doi.org/10.18231/j.jpmhh.2021.022
